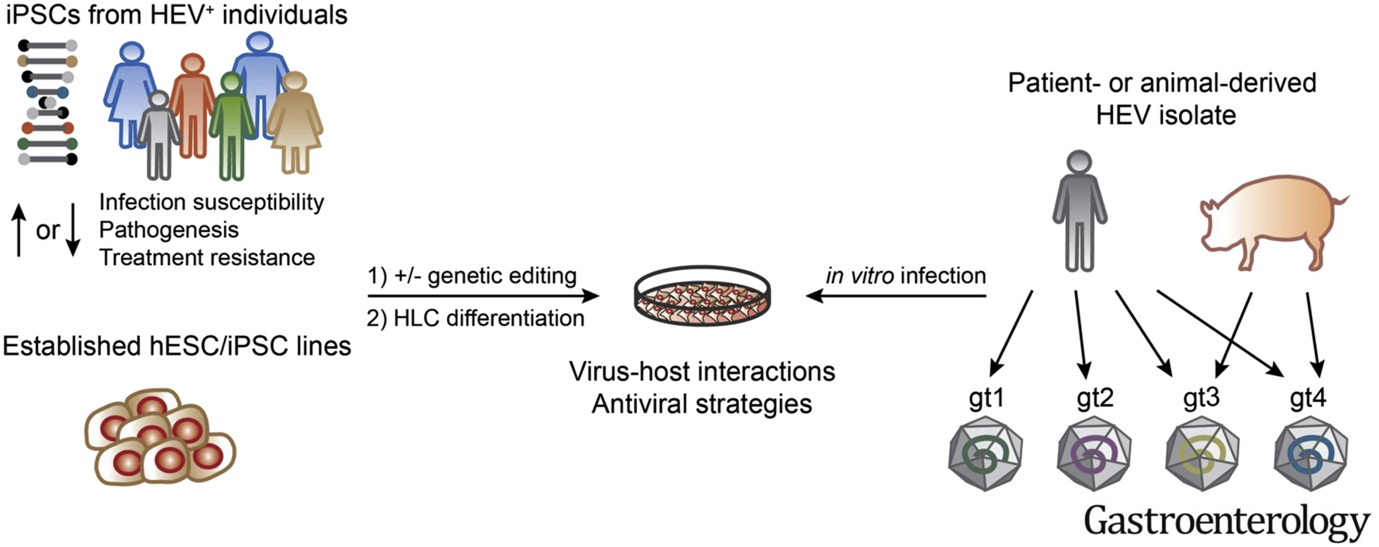
The heterogenous nature of hepatitis E virus (HEV) infections poses clinical challenges. Tropical HEV genotype (GT)1 & 2 infections can lead to acute liver failure, especially in pregnant women, while zoonotic HEV GT3 & GT4 infections can lead to acute-on-chronic liver failure in patients with underlying liver diseases. Major advances have been made by adapting the zoonotic GTs to propagate in human hepatoma cells. However, similar efforts to adapt GT1 & 2 isolates in cell culture have not been successful.
Here, Dr. Viet Loan Dao Thi and Dr. Xianfang Wu tell how they develop pluripotent stem cell-derived HEV culture models
In a recent study, we previously showed that hepatocyte-like cells (HLCs) differentiated from human embryonic and induced pluripotent stem cells (ESC/iPSC) support the entire HEV life cycle. Due to their primary nature, HLCs reflect better the cellular environment than conventionally used hepatoma cells. Importantly, we found that HLCs can be infected with natural isolates from all major human-infecting HEV GTs 1-4, therefore providing a unique reproducible and genetically tractable platform to study pan-genotype biology of non-adapted HEV isolates (Wu and Dao Thi et al. 2018, Gastroenterology). The capability to study replication of such non-adapted HEV isolates in tandem with autologous, patient-derived iPSCs enables the generation of personalized HEV infection models as a novel platform for testing personalized antiviral intervention strategies.
Apart from the possibility to differentiate stem cell systems to any desired HEV-permissive tissue, we can also take advantage of their plasticity to generate cells with polarity of increasing complexity. HEV is principally transmitted through the fecal-oral route and the polarity of cells in involved tissues, i.e the gut (intestinal epithelial cells) and the liver (hepatocytes), plays a critical role. Usually posing a barrier between the inside and outside of the body, both intestinal cells and hepatocytes have developed fine-tuned trafficking machineries to provide the uptake of nutrients on the one hand and the directional secretion of distinct cargoes on the other hand. In a simplified manner, HEV has to enter these cells from one side and leave from the other side.
In order to study determinants of HEV transmission, we recently developed a novel stem cell-based differentiation protocol that uses transwell filters to generate columnar polarized HLCs (Dao Thi et al. 2020, Nat Commun). We found that polarized HLCs secrete cargo directionally: Albumin, urea, and lipoproteins are secreted basolaterally, whereas bile acids are secreted apically. We further showed that HEV progeny particles are secreted basolaterally as quasi-enveloped particles and apically as naked virions, thus recapitulating essential steps of the natural infectious cycle in vivo.
With their ability to provide universally susceptible systems for personalized, complex, and at the same time isogenic infection models, we believe that ESC/iPSCs-derived culture systems have the potential to transform the future of HEV biology studies.
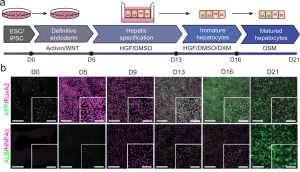
Figure adopted from Dao Thi et al. 2020, Nat Commun: a) Stem cell differentiation protocol on transwells to generate polarized HLCs. b) Representative immunofluorescence images of hESCs (day 0), definitive endoderm (day 5), hepatic progenitor (day 9 and 13), immature HLCs (day 16), and pol-HLCs (day 21). Cells were stained for immature hepatocyte markers AFP (green) and FoxA2 (magenta) or for mature hepatocyte markers ALB (green) and HNF4α (magenta). Scale bars = 500 μm/250 μm.
These studies were conducted in the laboratory of Charles M Rice and published in journals Gastroenterology and in Nature Communications in 2018 and 2020, respectively.
Read the original articles:
https://doi.org/10.1053/j.gastro.2017.10.041
https://doi.org/10.1038/s41467-020-15337-2