The hepatitis E virus (HEV) is a leading cause of acute hepatitis and is primarily transmitted via the fecal-oral route. Naked (nHEV) particles, which are highly infectious and critical for person-to-person transmission, are found in feces. When HEV buds from host cells, it acquires a membranous, host-derived envelope, forming quasi-enveloped HEV (eHEV). Although this envelope reduces the virus’s infectivity, it shields the capsid from neutralizing antibodies present in the blood of infected individuals.
Despite its global health importance, the cellular mechanisms governing HEV’s life cycle—particularly how the virus enters host cells—remain poorly understood. To address this, Fu et al. developed a high-content RNA-fluorescence in situ hybridization (FISH) imaging assay to track the entry pathways of both naked and quasi-enveloped HEV particles.
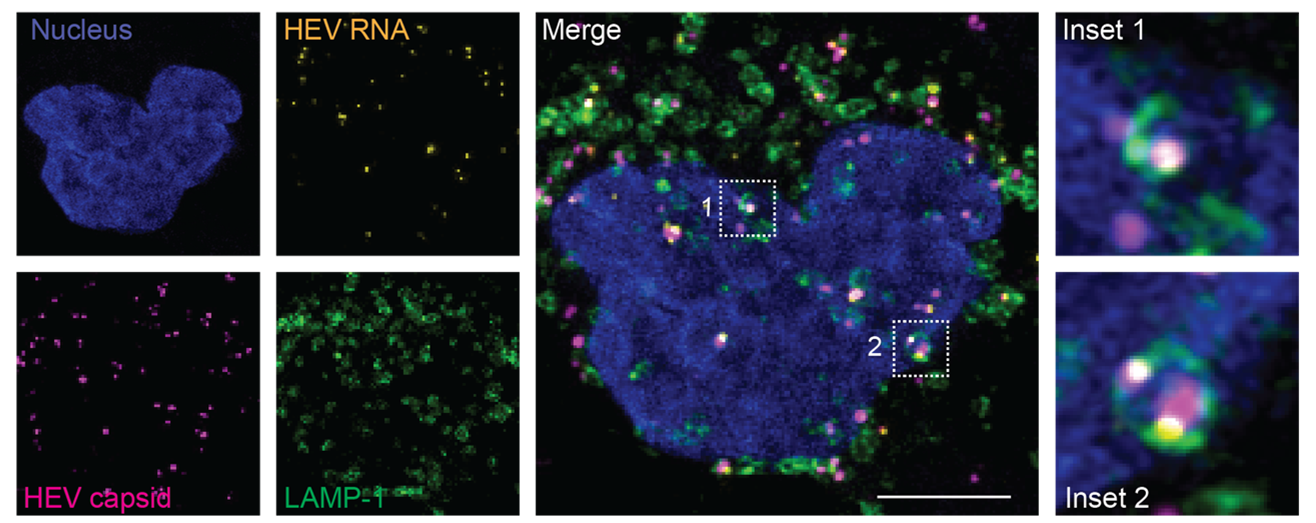
Previously, integrin α3 was proposed as an entry factor for naked HEV (nHEV). However, in a recent study by Fu et al., led by Dr. Viet Loan Dao Thi from Heidelberg University in Germany, the authors found that this particular alpha integrin is not expressed in cell lines highly permissive to nHEV infection. Instead, they identified integrin β1 (ITGB1), which can pair with various α-integrins, as the key receptor mediating nHEV entry. The study demonstrated that nHEV interacts with ITGB1 via a DGR motif, a binding motif found in several ITGB1 ligands. This interaction is essential for efficient binding and internalization of nHEV into host cells and directs the virus into Rab11-positive recycling endosomes.
In contrast, the researchers observed that eHEV particles do not bind to ITGB1 and instead enter cells through a conventional endocytic pathway involving Rab5a-positive early endosomes. They further found that both eHEV and nHEV particles rely on endosomal acidification and lysosomal proteases to facilitate infection, as these processes enable the release of the viral genome into the cytoplasm.
Read the full article: Nature Communications. 2025 Jun 26;16(1):5403. DOI: 10.1038/s41467-025-61071-y