Hepatitis E virus (HEV) is a quasi-enveloped, positive-sense single-stranded RNA virus classified within the family Hepeviridae, comprising various zoonotic and host-restricted strains. Recent studies indicate that HEV infection can progress to chronic disease, acute liver failure, and extrahepatic manifestations, suggesting complicated molecular mechanisms are involved in HEV pathogenesis.
During the life cycle of HEV, the ORF3 protein, plays an essential role. Although it is not directly involved in viral genome replication, ORF3 functions as a multifunctional protein which is indispensable for the release of viral particles. However, the precise molecular mechanisms by which ORF3 orchestrates the cytoskeleton to mediate viral trafficking and release remain poorly understood.
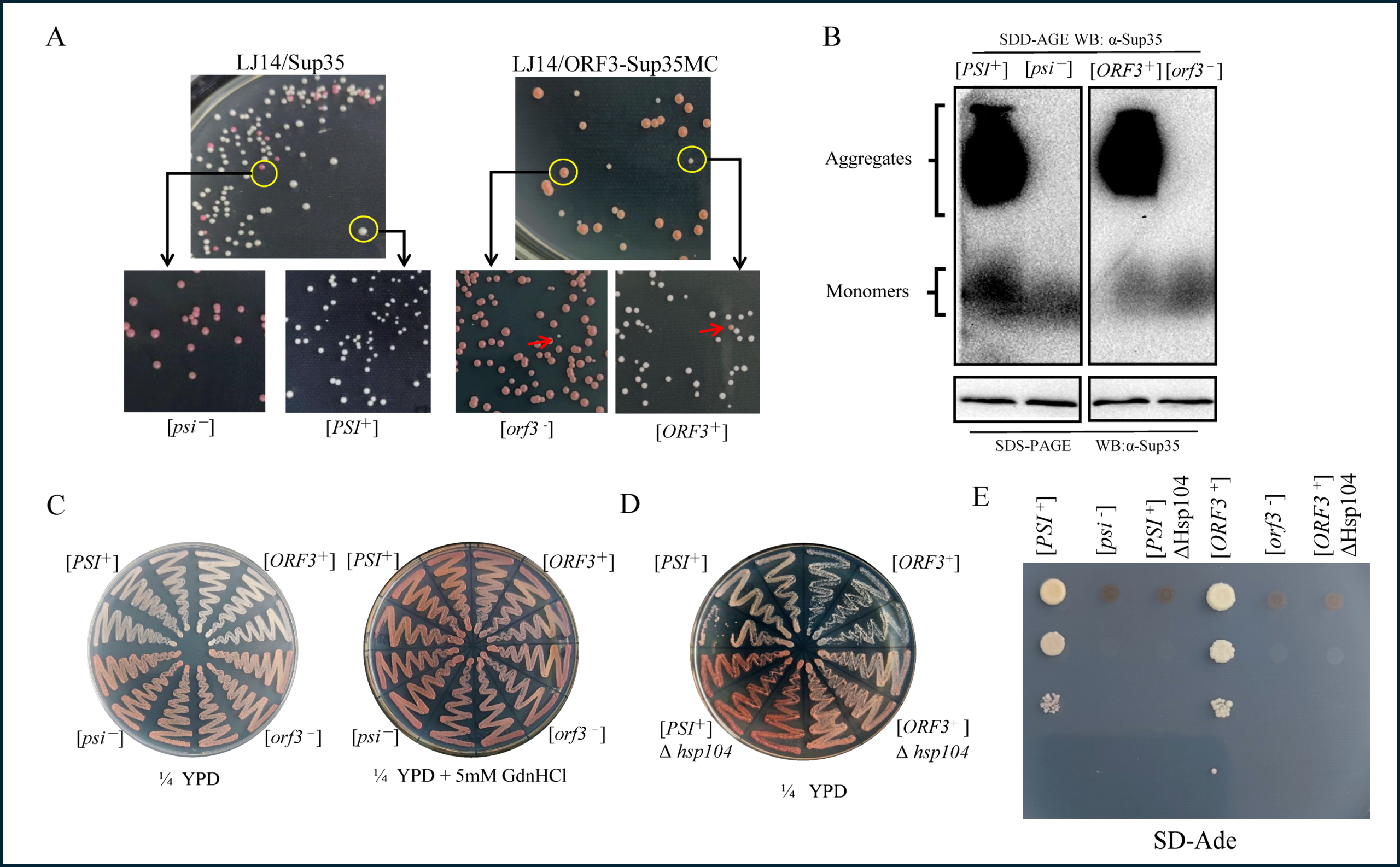
Recently, a collaborative study, led by Professor Yuchen Nan from the College of Veterinary Medicine and Professor Hongying Chen from the College of Life Sciences at Northwest A&F University demonstrated that the HEV-ORF3 protein exhibits typical prion properties in HEV-infected cells. It not only exists in a monomeric form but also assembles into SDS-resistant aggregates. In vitro assay further confirmed that these aggregates can act as a prion-like template to induce conversion and propagation of ORF3 monomers into aggregates. In yeast model, either the full-length ORF3 or its N-terminal domain could fully replace the functional domain of the canonical prion protein Sup35, genetically establishing its prion-like attributes. By employing reverse genetic system of HEV, the team also revealed that substituting phenylalanine at position 10 with serine (F10S) significantly impaired the aggregation capability of ORF3. This disruption consequently abolished ORF3’s function in stabilizing microtubules, ultimately blocking the trafficking and release of viral capsids.
In a Mongolian gerbil model, HEV mutant bearing F10S mutation in ORF3 exhibited attenuated virulence in vivo compared to wild-type HEV, as evidenced by reduced viremia levels, decreased viral shedding, and alleviated liver pathological damage in infected animals. Collectively, these findings elucidate a novel mechanism by which ORF3 regulates viral release and pathogenicity through its prion-like properties.
This discovery not only confirms that HEV-ORF3 is a prion-like protein which is involved in viral capsid trafficking and release, but also provides theoretical support for the scientific hypothesis that “The self-propagating properties of prion and prion-like proteins are widely existed in nature and play diverse roles in physiological process.”
The study was published in the Proceedings of the National Academy of Sciences (PNAS) on December 4, 2025. DOI: 10.1073/pnas.2511801122