Enterically transmitted hepatitis E (HE) shows worldwide distribution and follows distinct epidemiology in developing and developed nations. Hepatitis E virus (HEV), the etiological agent of HE exhibits ever expanding host range, complex transmission patterns and circulation of distinct genotypes in different geographies/species (1). Aforementioned HEV characteristics significantly influence HE diagnosis which can be done by detecting HEV genome in serum/feces using polymerase chain reaction or by serological detection of specific antigens/antibodies. Virus isolation is not feasible as HEV shows limited growth in tissue culture. Specific IgM antibodies in serum offer as most reliable and feasible HE diagnostic marker for detecting ongoing/recent HEV infections. Several research laboratory based/commercial immunoassays in ELISA, Western blot and immunochromatographic strip test formats have been reported. But an US Food and Drug Administration approved HE diagnostic gold standard test is not yet available. Evaluation of any new/refined serological assay relies on its comparison with available assays considered as reference/s. Varied performances of many HE serological assays has been reported predominantly from nations with developed or emerging economies and has restricted the understanding of true extent of HE.
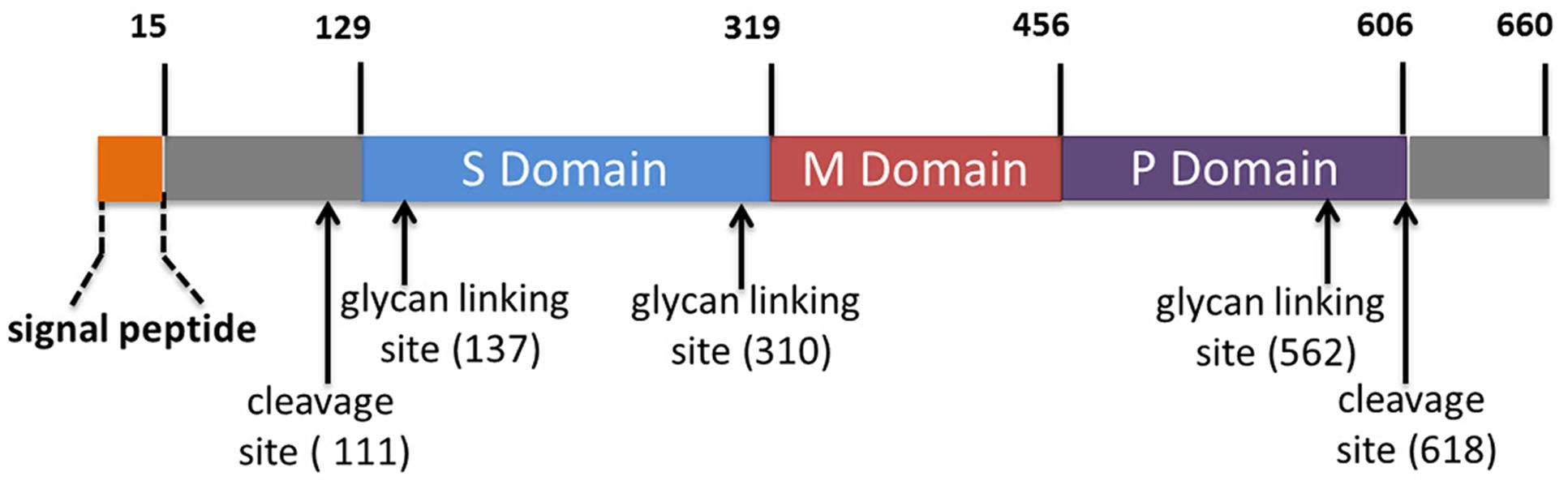
HE in its sporadic and epidemic forms continues to prevail as significant public health problem endemic across India and is often implicated in acute viral hepatitis (AVH) and acute liver failure cases (2). Rapid definitive differential diagnosis lies at the core of effective management of AVH outbreaks (3, 4). In this context, a recent study led by Dr. Tejaswini M. Deshmukh, Ms. Manisha T. Dudhmal and Dr. Kavita S. Lole evaluated performances of two in house and six commercial IgM detection enzyme linked immunosorbent assays (ELISAs) using sera collected from volunteers/acute hepatitis patients (n=716). The in house ELISAs were based on complete and truncated open reading frame 2 (ORF2) proteins containing neutralizing epitope/s region of genotype 1 HEV (ORF2p, 1-660 amino acid (a.a.) and T1NEp, 458-607 a.a., respectively). The commercial ELISAs included; Wantai (China), MP Diagnostics (MPD) (Singapore), DIA.PRO Diagnostics (Italy), MBS (Italy), abia (Germany) and ImmunoVision (USA). T1NE ELISA showed 97.0% Positive Percent Agreement (PPA), 99.4% Negative Percent Agreement (NPA) and 98.6% concordance (κ=0.97, P=0.0000) with ORF2 ELISA. ORF2, T1NE, Wantai and MPD ELISAs agreed on results for 88% of sera tested. Two percent sera showed reactivity in each combination of three and two of aforementioned four ELISAs. Remaining 8% sera were single ELISA reactive. PPA and NPA value ranges were 76.3-99.0% and 84.8-99.5%, respectively. Pairwise concordances between all the eight ELISAs ranged from 88.0-100% (κ: 0.74-1.00). Both the in house ELISAs agreed better with Wantai over MPD ELISA. Both ORF2 and T1NE ELISAs were equally efficient in diagnosing HEV infections. T1NEp proved to be an excellent tool in HE sero-diagnosis and can be feasibly used in the development of rapid tests. The study had some limitations wherein performances of DIA.PRO, MBS, abia and ImmunoVision ELISAs were evaluated using relatively a small sample subset, sensitivity of all the eight ELISAs in detecting specific IgM antibodies was not assessed beyond 45 days post infection/onset of symptoms and ability to detect HEV infections caused due to mammalian genotypes other than 1 was not tested. This study reiterated the need for extensive evaluation of available assays using defined global serum reference panels.
Read the full article (Appl Microbiol Biotechnol 2022.): https://rdcu.be/cZLiv
References:
- Purdy MA, Drexler JF, Meng XJ, Norder H, Okamoto H, Van der Poel WHM, Reuter G, de Souza WM, Ulrich RG, Smith DB and ICTV Report Consortium (2022) ICTV Virus Taxonomy Profile: Hepeviridae J Gen Virol 103:001778. https://doi.org/10.1099/jgv.0.001778
- Kumar T, Shrivastava A, Kumar A, Laserson KF, Narain JP, Venkatesh S, Chauhan LS, Averhoff F (2015) Viral Hepatitis Surveillance-India, 2011-2013. MMWR Morb Mortal Wkly Rep 64:758-762. https://doi.org/10.15585/mmwr.mm6428a3
- Chobe LP, Arankalle VA (2009) Investigation of a hepatitis A outbreak from Shimla Himachal Pradesh. Indian J Med Res 130(2):179-84. PMID: 19797816
- Tripathy AS, Das R, Chadha MS, Arankalle VA (2011) Epidemic of Hepatitis B with high mortality in India: association of fulminant disease with lack of CCL4 and natural killer T cells. J Viral Hepat 18: e415-e422 https://doi.org/10.1111/j.1365-2893.2011.01457