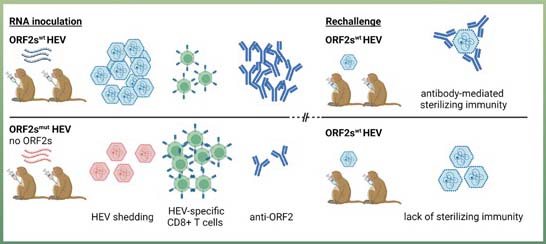
HEV has a single capsid protein encoded by its open reading frame (ORF) 2. Recent studies have shown that most of the ORF2 protein found in the circulation of HEV-infected patients is not associated with virions and therefore may function as an immunomodulatory molecule (1, 2) . The role of this secreted form of ORF2 (ORF2s) in vivo remains elusive. In a recent study (3), Ralfs et al. reported that selective silencing of ORF2s and not the capsid form of ORF2 (ORF2c) does not abolish HEV infection in rhesus macaques, but does alter adaptive immune responses.
The biogenesis of the ORF2s protein remains controversial. One theory is that ORF2s and the ORF2c are both derived from the same, full-length ORF2 protein but are routed to different compartments due to a dual membrane orientation of the signal sequence present at the N terminus of the protein. An alternative explanation is that ORF2s and ORF2c are two distinct proteins produced via leaky scanning (2). The study by Ralfs et al. pursued the latter theory and introduced mutations to specifically eliminate ORF2s expression. The impact of silencing ORF2s on HEV replication and immunity was assessed in rhesus macaques. Intrahepatic injection of this mutant HEV RNA into two animals resulted in successful infection. However, the replication was attenuated when compared to wild-type HEV RNA that was similarly injected into the liver of two other animals. In all cases, the fecal viral shedding lasted about 3-4 weeks, coinciding with an increase in HEV-specific antibody and T cell responses. The ORF2-specific antibody titers and CD8+ T cell response were both lower in the absence of ORF2s and failed to protect those animals against reinfection. Interestingly, the ORF1-specific CD8+ T cell response appeared to be enhanced in the absence of ORF2s, possibly facilitating virus clearance (3).
This study represents the first attempt to understand the role of ORF2s in HEV infection in vivo and offers several insights into the HEV life cycle and immunobiology. First, it provides additional evidence for the leaky scanning model of ORF2s biogenesis. Second, the study demonstrates that the production of ORF2s is not essential for HEV infection in vivo. Third, the results indicate that ORF2s production contributes to HEV-specific antibody and T cell responses, which are important for protection against reinfection. Finally, although speculative, the reduced ORF1-specific CD8+ T cell response in the absence of ORF2s suggests that ORF2s expression may exert a negative role in class I antigen presentation in HEV-infected cells.
Many questions remain. It is unclear how silencing ORF2s attenuated HEV replication in rhesus macaques. To ensure ORF2s silencing, five point-mutations were introduced into the HEV genome which may have impaired virus replication. Future study with less changes in the viral genome to eliminate ORF2s expression would provide an answer to this question. While this study shows that ORF2s is not essential for HEV infection, its impact on innate and adaptive immunity has not been specifically addressed. Given its antigenic overlap with the ORF2c, it has been proposed that ORF2s functions as an immunological decoy (2). How the absence of ORF2s alters antibody repertoire and function requires further study. Serum ORF2s levels are higher in chronic infection than in acute infection and serum therapy failed to reduce viral load in a chronic hepatitis E patient (4). It will be interesting to test if genetic silencing of ORF2s would reduce viral fitness in immunosuppressed individuals, and if reducing the serum ORF2s protein level in chronically infected patients would yield a more effective response to antibody-based therapy.
Read the full article (Hepatology. 2023 Apr 27. DOI: 10.1097/HEP.0000000000000421).
Key references
- Montpellier C, Wychowski C, Sayed IM, Meunier JC, Saliou JM, Ankavay M, Bull A, Pillez A, Abravanel F, Helle F, Brochot E, Drobecq H, Farhat R, Aliouat-Denis CM, Haddad JG, Izopet J, Meuleman P, Goffard A, Dubuisson J, Cocquerel L. 2018. Hepatitis E Virus Lifecycle and Identification of 3 Forms of the ORF2 Capsid Protein. Gastroenterology 154:211-223 e8.
- Yin X, Ying D, Lhomme S, Tang Z, Walker CM, Xia N, Zheng Z, Feng Z. 2018. Origin, antigenicity, and function of a secreted form of ORF2 in hepatitis E virus infection. Proc Natl Acad Sci U S A 115:4773-4778.
- Ralfs P, Holland B, Salinas E, Bremer B, Wang M, Zhu J, Ambardekar C, Blasczyk H, Walker CM, Feng Z, Grakoui A. 2023. Soluble ORF2 protein enhances HEV replication and induces long-lasting antibody response and protective immunity in vivo. Hepatology doi:10.1097/HEP.0000000000000421.
- Ankcorn M, Gallacher J, Ijaz S, Taha Y, Harvala H, Maclennan S, Thomson EC, Davis C, Singer JB, da Silva Filipe A, Smollett K, Niebel M, Semple MG, Tedder RS, McPherson S. 2019. Convalescent plasma therapy for persistent hepatitis E virus infection. J Hepatol 71:434-438.