Hepatitis E virus (HEV), a single-stranded positive-sense RNA virus, belongs to the Hepeviridae family and is the causative agent of hepatitis E. HEV is distributed worldwide and is most prevalent in developing countries; however, the seropositivity rate in many developed countries can exceed 10%, which is a significant concern.
Since the discovery of HEV, extensive efforts have been made to establish culture models for in vitro recapitulation; however, progress has been limited. To date, only one cell-adapted HEV strain, Kernow-C1/p6, has demonstrated replication in hepatoma cell lines with moderate efficiency. Widely used culture models, including Huh7, Huh7-Lunet BLR, HepG2/C3A, PLC/PRF/5 and HepaRG liver cancer cell lines, as well as A549 lung adenocarcinoma cells, and conventional two-dimensional (2D) monolayer systems, exhibit low infection efficiency with clinical strains because of poor similarity to hepatic tissue development and physiology. Consequently, studies have explored induced pluripotent stem cell (iPSC)-derived hepatocyte-like cells (HLCs) or neurons, as well as cultures derived from adult primary human Sertoli cells and human intestinal cells, for HEV propagation. Recently, three-dimensional (3D) hepatic epithelial organoids from primary foetal or adult biopsies were established and found to be susceptible to cell-adapted HEV strains in vitro. However, these models contain only one or two sub-lineages (eg, liver organoids typically consist of hepatocytes and/or cholangiocytes), limiting their utility for investigating HEV infection in more physiologically intact systems that include non-epithelial supportive lineages, such as mesenchymal or immune cells. Moreover, although HEV is hepatotropic, extrahepatic replication and manifestations—including neurological injury and intestinal infection—have been frequently reported in patients. Therefore, a robust in vitro platform for investigating extrahepatic infection pathogenesis is urgently needed.
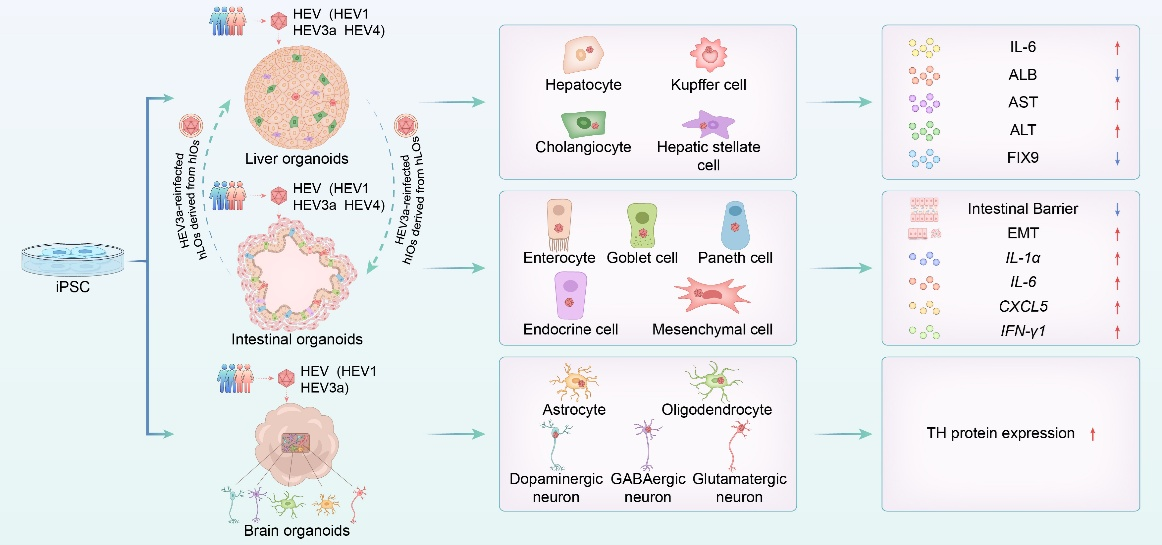
Recently, the US Food and Drug Administration and the National Institutes of Health (NIH) jointly promoted the adoption of ‘New Approach Methodologies’, including organoid-based technologies, to progressively replace traditional animal testing. Addressing this need, Dr. Lin Wang and Dr. Tianxu Liu in Peking University, Beijing, China, collaborated with Dr. Weijin Huang and Prof. Di Wu, systematically developed human 3D multilineage liver, small intestinal and brain organoids from iPSCs and co-cultured them with clinical HEV strains. These multilineage mini-organs effectively supported HEV infection and reproduced pathophysiological features observed in patients, thereby providing a valuable tool for in vitro research on wild-type HEV.
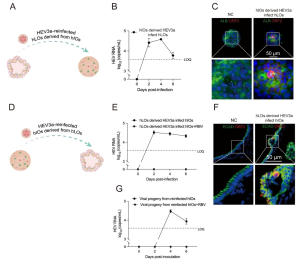
The hLOs-hIOs system modelled the sequential gut-liver-gut infection cycle of HEV.
Notably, they established a bidirectional transmission cycle between intestinal and liver organoids. Supernatant from infected human intestinal organoids (hIOs) successfully reinfected human liver organoids (hLOs), mirroring the natural gut-to-liver route. Conversely, supernatant from infected hLOs established productive infection in hIOs, recapitulating in vivo liver-to-gut transmission. This cross-infection model reinforces the intestine as an extrahepatic replication site for HEV, potentially facilitating viral dissemination and faecal–oral transmission. Although antiviral treatment suppressed replication, progeny viruses from secondary infections remained infectious, demonstrating sustained dissemination across organoid types.
In conclusion, they demonstrated that human liver, small intestinal and brain organoids support the complete HEV life cycle, including replication and secretion of infectious progeny from clinical isolates. The multicellular complexity of these systems provides a powerful platform for dissecting pathogenic mechanisms in the liver, intestine and brain, particularly in understudied extrahepatic sites. The study has been online published in Gut, in October 2025. DOI: 10.1136/gutjnl-2025-336105