Hepatitis E virus (HEV) is a leading global cause of acute viral hepatitis, posing a severe threat to public health. The infection is especially dangerous for vulnerable populations: pregnant women face mortality rates of up to 25% due to acute liver failure, while immunocompromised individuals often develop chronic infections that can progress to cirrhosis. Despite this significant burden, no specific antiviral drugs have been approved for HEV. Current treatments such as ribavirin and interferon-alpha are limited by serious side effects, resistance, and contraindications in key patient groups. This therapeutic gap highlights the urgent need for innovative and safe antiviral strategies.
In the search for novel targets, guanine-rich nucleic acid structures known as G-quadruplexes (G4s) have emerged as promising antiviral candidates. Initially identified in human DNA, G4s regulate key processes such as replication and transcription in various viruses, including hepatitis B and C viruses, SARS-CoV-2, and HIV. Although G4s are well-studied in other pathogens, their role in HEV has remained unexplored—until now.
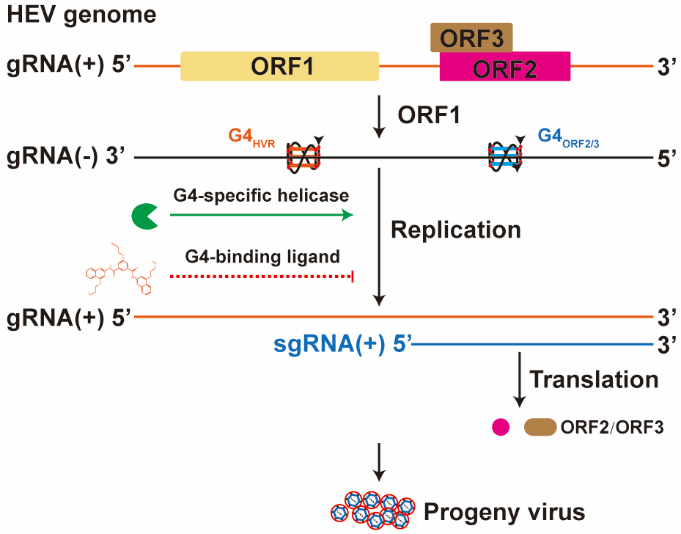
In a recent breakthrough, a team led by Professors Wenshi Wang and Hongbo Guo from Xuzhou Medical University in China identified two highly stable G4 motifs—G4HVR and G4ORF2/3—within the negative-sense RNA of HEV. These structures form stable parallel conformations that can be further stabilized by the G4-binding ligand pyridostatin (PDS). Cellular experiments showed that PDS-locked G4s function as potent “off switches”, significantly repressing viral gene expression. Moreover, in HEV infection models, PDS treatment robustly inhibited both viral RNA synthesis and ORF2 capsid protein production.
The study’s robustness was underscored by rigorous mechanistic validation: the antiviral effect, attributable specifically to G4 stabilization, was replicated by a structurally distinct G4-targeting compound (TMPyP4) but not by an inactive control. This confirms a direct and targetable mechanism, laying a solid foundation for RNA-directed therapeutic development against HEV. By uncovering this previously unrecognized regulatory layer, the work opens a novel pathway toward safe and effective antiviral strategies for a disease in urgent need of treatment options.
Reda the full article: ACS Infect Dis. 2026 Jan 28. DOI: 10.1021/acsinfecdis.5c01069